A simple and rapid method for chiral separation of amlodipine using dual chiral mobile phase additives
Abstract
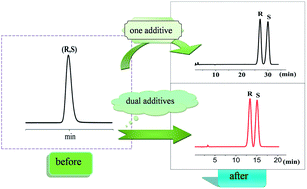
A simple, rapid and sensitive method for chiral separation of the amlodipine racemate was developed. (R)- and (S)-amlodipine enantiomers were separated by achiral HPLC, utilising dual chiral mobile phase additives, within 16 min, which was much faster than the previous similar studies. The method was established on an achiral WondaSil C18 column, using methanol–water (45 : 55 (v/v), pH 2.5) containing 7.5 mmol L−1 sulfobutylether-β-cyclodextrin (SBE-β-CD) and 0.3 mmol L−1 polyethylene glycol-20 000 (PEG-20M) as the mobile phase. Effects of additives and concentration, content of methanol and pH on the enantioseparation were investigated. The method was validated for linearity, repeatability, limits of detection (LOD) and limits of quantification (LOQ). The calibration curve (R(S)2 = 0.9998) was constructed in the range of 10–500 μg mL−1 for (S)-amlodipine. Repeatability (n = 6) showed less than 2% relative standard deviation (R.S.D.). LOD and LOQ were found to be 0.032 and 0.106 μg mL−1 for (S)-amlodipine. This method was flexible, simple and economically advantageous over the use of a chiral stationary phase, and was successfully applied to the determination of the amlodipine enantiomers in a commercial pharmaceutical tablets study.

 Please wait while we load your content...
Please wait while we load your content...