Detection of ochratoxin A based on the use of its diastereoisomer as an internal standard
Abstract
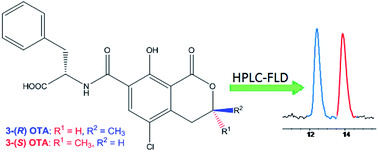
The excellent separation achieved by HPLC of the diastereoisomers 3-(R) and 3-(S) of ochratoxin A and their identification by fluorimetric detection has prompted the development of a new method for the detection of ochratoxin A, one of the most abundant food-contaminating mycotoxins in the world. The new method is based on the use of N-[(5-chloro-3,4-dihydro-8-hydroxy-3-(S)-methyl-1-oxo-1H-2-benzopyran-7-yl)carbonyl]-L-phenylalanine as an internal standard. This compound was synthesized by a coupling reaction between commercially available phenylalanine and ochratoxin α. The effectiveness of the method is confirmed by the very low limit of detection and quantitation values achieved for the detection of ochratoxin A in two fortified matrixes (0.002 and 0.005 μg L−1, respectively, in wine; 0.029 and 0.072 μg kg−1, respectively, in flour) and is further supported by the observed accuracy values. The new approach meets the requirement for the detection of subpicogram amounts of contaminating toxins in complex matrices such as foods. Moreover, the method is affordable to any laboratory because it is based on relatively inexpensive separation and detection methodologies.

 Please wait while we load your content...
Please wait while we load your content...