Stability-indicating thin-layer chromatographic method for determination of darunavir in complex darunavir–β-cyclodextrin in the presence of its degradation products†
Abstract
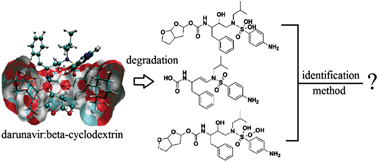
Darunavir (DRV), a protease inhibitor used in the treatment of HIV infection, presents low water solubility and poor bioavailability. Therefore, the complexation of DRV with β-cyclodextrin (β-CD) was performed. This drug is not described in official compendiums. A simple, selective and inexpensive stability-indicating thin-layer chromatographic (TLC) method for the determinations of DRV in complex DRV–β-CD and its degradation products was developed. The TLC method employs aluminum plates precoated with silica gel 60F-254 as the stationary phase and purified water and methanol, 70 : 30 (v/v) adjusted to pH 2.4 with glacial acetic acid as the solvent system to provide spots for DRV (Rf = 0.66) and its degradation products in acidic (Rf = 0.73 and 0.76), basic (Rf = 0.53) and oxidative (Rf = 0.71, 0,75 and 0.84) media. The chromatogram was visualized in a UVA chamber at 365 nm. HPLC analysis was performed on a Waters HPLC system, Phenomenex CN Luna (250 × 4.6 mm) column and mobile phase consisting of water + 0.1% glacial acetic acid and acetonitrile + 0.1% glacial acetic acid in the ratio 60 : 40 (v/v) at a flow rate of 1.0 mL min−1 and 268 nm for the separation of DRV (tR = 7.3) and its degradation products in acidic (tR = 5.1 and 6.7 min), basic (tR = 7.8 min) and oxidative (tR = 5.1, 5.5 and 6.7 min) media. DRV–β-CD was subjected to acid and alkali hydrolysis and oxidation and analyzed by the proposed methods in the presence of its degradation products, which were identified by LC-MS. As the methods could separate DRV from the degradation products, these techniques can be employed as indicative stability methods and can be effectively applied in quality control of DRV complexed to β-CD.

 Please wait while we load your content...
Please wait while we load your content...