Quantification of total serum transferrin and transferrin sialoforms in human serum; an alternative method for the determination of carbohydrate-deficient transferrin in clinical samples†
Abstract
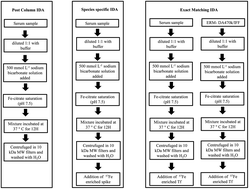
Carbohydrate deficient transferrin (CDT) is a biochemical marker for congenital disorders of glycosylation (CDG), chronic alcohol consumption, and forensic medicine diagnosis. However it is necessary to take into account that CDT is not a single molecular entity but refers to a group of transferrin (Tf) sialoforms (asialo-, monosialo-, disialo- and occasionally trisialo-Tf). A number of methods have been developed for CDT measurement based on different analytical techniques and principles without harmonization or calibration to a reference method or a certified reference material, hampering understanding of the diagnostic value of CDT and its routine use. Thus, it is unquestionable that there is a need for a reference material which permits the accurate and precise determination of each individual Tf sialoform which could serve as a universal calibrator for routine immunologic methods used in clinical laboratories. In this work, we describe highly sensitive ICP-MS isotope dilution analysis (IDA) methods for the separation and quantification of the different Tf sialoforms in human serum. The methodology was applied to measure the concentration of each sialoform of Tf and the total concentration of Tf in the NIST Standard Reference Material (SRM) 909c human serum. Additionally, two clinical laboratory control serums utilized for routine analysis of CDT were also analyzed. The separation of the sialoforms was achieved by anion exchange chromatography. The two IDA techniques applied for the quantification of the Tf sialoforms were: (a) post-column IDA and (b) species-specific IDA with the total concentration of Tf calculated by these two being the sum of the individual sialoforms. A third IDA technique, exact matching IDA was applied to determine the total concentration of Tf in SRM 909c. All the Tf measurements were validated with the ERM-DA470-IFF human serum (IRMM, Geel, Belgium) certified for total Tf. Finally, the identification of each Tf sialoform previously separated in the serum (SRM 909c) was carried out by LC/MS/MS.
- This article is part of the themed collection: Clinical Diagnostics

 Please wait while we load your content...
Please wait while we load your content...