Pharmacokinetic study of liquiritin in rat serum using molecularly imprinted solid-phase extraction combined with RP-HPLC
Abstract
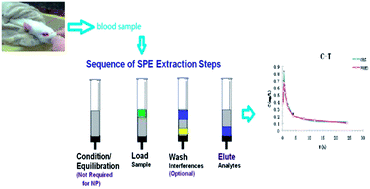
A method using molecularly imprinted solid-phase extraction combined with RP-HPLC-UV was developed for determination of liquiritin (LQR) in rat plasma after oral administration of Compound Liquorice Tablets (CLTs). To this end, molecularly imprinted polymers were prepared by in situ polymerization, using LQR as the template, acrylamide as the functional monomer, and ethylene glycol dimethacrylate as the crosslinker, whose solid-phase extraction properties for liquiritin were evaluated by HPLC-UV. The enrichment and extraction recovery were investigated in the study after the extraction procedure was optimized. The recovery was larger than 85.0% and the RSD was less than 10.0%. The calibration curve was expressed as y = 16 004x − 1.864 (r = 0.996) with a linear range of 0.007–1.683 μg mL−1. Besides, the Tmax was 0.5 h for liquiritin and the Cmax was 0.8 μg mL−1 after oral administration of compound liquorice tablets. In addition, the pharmacokinetic parameters were calculated using a two-compartment model. The results indicated that the monolithic polymer pipette presented a good extraction efficiency for liquiritin. At the same time, the results indicated that the index components could basically reveal the pharmacokinetic behavior. This study was aimed to explore an approach that could be applied to promulgate the pharmacokinetics and provide more information for clinical applications.

 Please wait while we load your content...
Please wait while we load your content...