Simple and rapid determination of salsalate in pharmaceutical preparations by chemiluminescence
Abstract
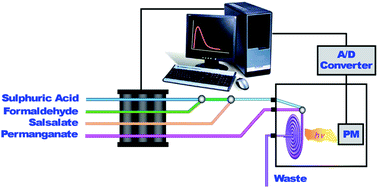
The chemiluminescent behaviour of salsalate when reacted with a common oxidant, potassium permanganate in different acidic media, using the stopped-flow technique in a continuous-flow system is described. The optimum chemical conditions for the chemiluminescence emission were investigated. A weak CL emission was found to be emitted during the oxidation of this drug with potassium permanganate in an acidic solution. The effect of common emission enhancers, organized systems and metals ions was investigated. The parameters selected were 4.0 mol L−1 sulfuric acid, 1.5 mmol L−1 permanganate and 0.25 mol L−1 formaldehyde. Three quantitative parameters adjustable via software settings, one of them typically a kinetic parameter, such as the rate of the light-development reaction, and the other conventional parameters, such as maximum emission intensity and total emission area, were used to obtain linear calibration graphs with each measurement parameter. The calibration graphs were linear for the concentration range from 25 to 250 ng mL−1. The detection limits, according to Clayton et al., ranged from 2.85 to 15.19 ng mL−1. The present chemiluminescence procedures were applied to the determination of salsalate in a Spanish pharmaceutical formulation, with excellent recovery, as the determination is free from interference from common excipients present in the formulation.

 Please wait while we load your content...
Please wait while we load your content...