A graphene oxide-based immobilized PNGase F reagent for highly efficient N-glycan release and MALDI-TOF MS profiling
Abstract
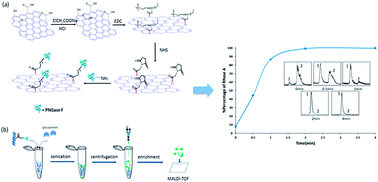
Protein N-glycosylation plays an important role in a variety of biological processes, and unusual changes in glycan structures are often correlated with various pathological states. Therefore, N-glycan profiling not only plays a vital role in the mechanism study of biological processes but also contributes to the discovery of biomarkers for early disease diagnosis. However, the conventional in-solution release of N-glycans by free endoglycosidases, such as peptide N-glycosidase F (PNGase F), suffers from several drawbacks, including prolonged incubation time, possible incomplete digestion and high cost due to the non-reusability of the enzyme. Herein, we report a novel graphene oxide (GO)-based immobilized PNGase F reagent for fast and highly efficient N-glycan release and glycoform profiling of glycoproteins. To create this reagent, PNGase F was attached to the GO surface via carbodiimide-activated amidation. The ultra-high surface area of GO allows for high loading with PNGase F and significantly facilitates the deglycosylation process. Highly efficient N-glycan release of ribonuclease B and asialofetuin was achieved with this reagent within 2 min. Furthermore, the successful application of our GO-based immobilized PNGase F in the N-glycan release of the human plasma glycoproteome was demonstrated by reproducible N-glycan identification using MALDI-TOF MS.

 Please wait while we load your content...
Please wait while we load your content...