Fabrication of a TCEP-immobilised monolithic silica microchip for reduction of disulphide bonds in proteins
Abstract
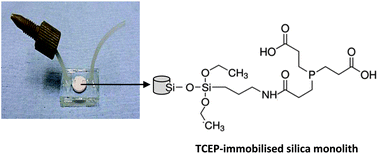
Protein identification by mass spectrometry forms the cornerstone of proteomics. Commonly, the identification of proteins is based on digestion of proteins into peptides using proteolytic enzymes. Cleavage of the disulphide bonds in proteins before enzymatic digestion and mass spectral analysis is important in order to facilitate the accessibility of the enzyme to cleave the proteins into peptides. As a result, the protein sequence coverage will be increased. In this study, a novel approach for immobilisation of the reducing reagent on the surface of a silica-based monolith in order to use it for reduction of disulphide bonds in proteins was successfully developed. This was carried out by silanisation of the surface of the silica-based monolith with (3-aminopropyl)triethoxysilane (APTES), followed by immobilisation of the reducing reagent, tris(2-carboxyethyl)phosphine hydrochloride (TCEP) on the surface of the amino-bonded silica monolith. The fabricated monolith was characterised using SEM analysis, EDX analysis, IR spectroscopy, and BET model. The performance of the fabricated glass microchip containing the TCEP-immobilised silica monolith to reduce the disulphide bonds in proteins was checked by injection of 100 μL of denatured insulin inside the microchip using a syringe pump at a flow rate of 10 μL min−1, followed by sealing both ends of the ETFE tubes with Blu-Tak. The microchip was kept in a humidified chamber for 30 min at 60 °C. After the reduction reaction, the reduced cysteine residues were alkylated with IAA at 60 °C for 30 min, followed by using a MALDI-TOF-MS instrument for qualitative confirmation. The results show that the fabricated microchip-based silica monolith has the ability to reduce disulphide bonds in insulin. In addition, the method is simple, reduces the risk of contamination, and results in lower amounts of the sample and reagents compared with the conventional techniques for proteomics sample preparation. A future study investigating reduction of the disulphide bonds in proteins from a real sample using this new microfluidic device would be very interesting.

 Please wait while we load your content...
Please wait while we load your content...