Retracted Article: Determination of silk fibroin secondary structure by terahertz time domain spectroscopy
Abstract
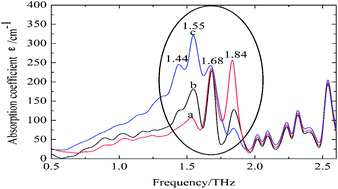
Silk fibroin membranes with different secondary structures were fabricated, and subsequently employed as an ideal system model for the investigation into the conformational transition of native proteins by terahertz time domain spectroscopy (THz-TDS). In comparison to conventional Fourier Transform Infrared (FTIR) Spectroscopy, THz spectroscopy presents distinct features for each kind of secondary structure with narrower bandwidths. Based on the theoretical calculation, absorption features observed in the range of 2.0–2.6 THz may be attributed to intramolecular modes of peptide chain. Three bands centered at 1.84 THz, 1.68 THz, and 1.55 THz are attributed to random coil, α-helix, and antiparallel β-pleated sheet, respectively. The results indicate that THz-TDS presents great potential as a complementary method to FTIR in determining the secondary structure of silk fibroin.

 Please wait while we load your content...
Please wait while we load your content...