Sensitive and selective colorimetric detection of Cu2+ in aqueous medium via aggregation of thiomalic acid functionalized Ag nanoparticles
Abstract
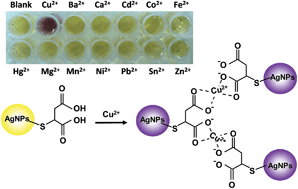
A simple and effective colorimetric method for determination of Cu2+ in real samples was developed. In this method, thiomalic acid functionalized silver nanoparticles (TMA-AgNPs) were prepared and changes in solution color, induced by the aggregation of TMA-AgNPs in the presence of Cu2+, were employed for quantitative analysis. The surface plasmon resonance (SPR) band of our synthesized TMA-AgNPs was located at 392 nm and shifted to a longer wavelength after aggregation due to the interactions between carboxylate and Cu2+. A band intensity ratio of A455/(A392 − A455) was constructed and used to correlate with the concentration of Cu2+. A linear relationship was found with a linear response up to 50 nM of Cu2+. Due to the formation of a stable carboxylate Cu2+ complex, highly sensitive detection of Cu2+ was achieved with the estimated detection limit approaching 1 nM. Moreover, the formation of the stable complex leads to high selectivity in the detection of Cu2+, which was verified by examination of 12 other metal ions. In the detection of Cu2+ in real samples, results indicated that our proposed method is simple, sensitive and selective for application in such measurements.

 Please wait while we load your content...
Please wait while we load your content...