Cyclic-AMP-dependent protein kinase (PKA) activity assay based on FRET between cationic conjugated polymer and chromophore-labeled peptide†
Abstract
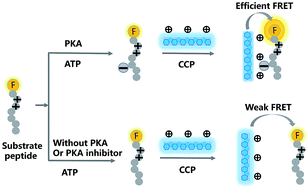
A sensitive fluorescence turn-on biosensing platform for protein kinase activity assay has been developed based on fluorescence resonance energy transfer (FRET) between a fluorophore labeled peptide and a water soluble cationic conjugated polymer (CCP). The CCP-based assay is based on the electrostatic interaction between the peptide and the CCP. The FRET efficiency will change with the changing charges around the peptide after phosphorylation. The feasibility of this method has been demonstrated by sensitive measurement of the activity of cAMP-dependent protein kinase (PKA) with a low detection limit (0.3 mU μL−1). Based on its simple mechanism, this assay is also sensitive and robust enough to be applied to the evaluation of PKA inhibitor H-89. The IC50 value, the half maximal inhibitory concentration, was 40 nM. Furthermore, our method has excellent selectivity. CCP-based assay is sensitive, versatile, cost-effective and easy to operate, so, this method is a promising candidate for kinase activity assay and inhibitor screening.

 Please wait while we load your content...
Please wait while we load your content...