Selective and sensitive detection of acetylcholinesterase activity using denatured protein-protected gold nanoclusters as a label-free probe†
Abstract
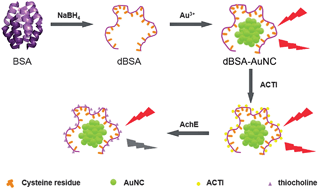
Based on the fluorescence quenching of novel denatured protein-protected gold nanoclusters, a label-free detection method of acetylcholinesterase (AChE) activity has been developed. Using denatured bovine serum albumin (dBSA), in which 35 cysteine residues can interact polyvalently with Au nanoclusters (AuNCs) as a stabilizing agent, water-soluble and stable fluorescent gold nanoclusters were synthesized. The fluorescence of the AuNCs was quenched by thiocholine that was produced from the AChE hydrolysis of S-acetylthiocholine iodide (ACTI) to detect the AChE activity. The linear range of the method was 0.005–0.15 U mL−1. The limit of detection (LOD) was 0.02 mU mL−1. Other enzymes and metal ions, i.e., GPT, γ-GT, GOx, K+, Ca2+ and Na+, showed minimal interference. Using the fluorescence probe, satisfactory results for the detection of the AChE activity in human serum were obtained.

 Please wait while we load your content...
Please wait while we load your content...