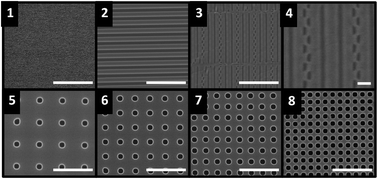
It is well documented that bacterial adhesion to surfaces is mediated by the physical and chemical properties of the substrate, as well as the surface characteristics of the organism. Topographical features that limit cell–surface interactions have been shown to reduce surface colonization and biofilm formation. In this study, bacterial attachment to medically relevant materials was evaluated. Our data show that Escherichia coli attachment to glass, silicone, and titanium surfaces was most affected by the surface energy of these materials, as determined by water contact angle. The inherent roughness of the surface, however, was not correlated with cell attachment density. To study the effect of engineered surface roughness on bacterial attachment, topographical features, including arrays of holes and repeating lines/trenches, were formed from silicon wafers and then used as a template to imprint silicone-based polydimethylsiloxane (PDMS). Patterned silicone surfaces were then used in static and microfluidic flow-based experiments to evaluate cellular settlement and attachment. Cell attachment was observed to be strongly dependent upon the topographical features under both static and microfluidic flow conditions. The highest attachment density was observed on flat, un-patterned surfaces, while linear patterned surfaces showed greatly reduced cell attachment. Moreover, surfaces consisting of arrays of holes further reduced cell attachment as compared to linear patterns. These results demonstrate that the size, spacing, and shape of surface features play a significant role in cell–surface attachment and provide insight for the design of surfaces with antifouling properties.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...