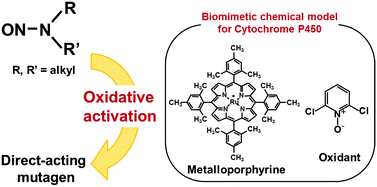
Ruthenium porphyrin and oxidant convert N-nitrosodialkylamines into direct-acting mutagen in the Ames assay
Abstract
The Ames assay is used for short-term screening of
* Corresponding authors
a Kyoritsu University of Pharmacy, Shibakoen 1-5-30, Minato-ku, Tokyo 105-8512, Japan
b
Faculty of Pharmaceutical Sciences, Tokyo University of Science, Yamazaki 2641, Noda-shi, Chiba 278-8510, Japan
E-mail:
inami@rs.noda.tus.ac.jp
Fax: +81-4-7121-3641
Tel: +81-4-7121-3641
The Ames assay is used for short-term screening of

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
