Bitrialkylsilylethynyl thienoacenes: synthesis, molecular conformation and crystal packing, and their field-effect properties†
Abstract
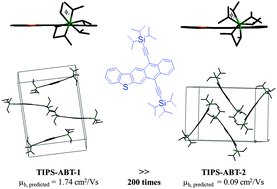
In order to study the influence of molecular conformation on the packing mode of single crystals, four bitrialkylsilylethynyl thienoacenes, TIPS–ABT, TMS–ABT, TIPS–CABT and TMS–CABT, were synthesized and characterized. Since there are different conformations arising from the rotation of the isopropyl groups, two types of single crystals of the thienoacene TIPS–ABT were successfully grown, and the related quantum-chemical calculations predict that in theory they have significantly different hole mobilties (μh). For example, the μh of TIPS–ABT-1 is 1.74 cm2 V−1 s−1, a value nearly two hundred times larger than that of TIPS–ABT-2 (0.09 cm2 V−1 s−1), when the reorganization energy is obtained at the B3LYP/6-31+G(d) level. The results demonstrate the important influence of molecular conformation on the mode of crystal packing, and theoretically show the importance of organic semiconductor conformational control on the charge mobility. The thin film FET devices based on the four thienoacenes were prepared via the vacuum-deposit method. The TIPS–CABT-based devices afford hole mobilities of up to 0.012 cm2 V−1 s−1 with a current on–off ratio of 106.

 Please wait while we load your content...
Please wait while we load your content...