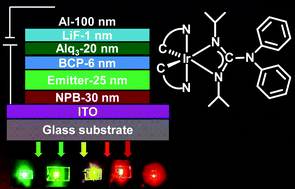
We report the synthesis, structure, and electrophosphorescence properties of a series of heteroleptic iridium(III) complexes with various cyclometalated (C⁁N) ligands based on the sterically demanding guanidinate ancillary ligand. The iridium(III) complexes contain two cyclometalated (C⁁N) ligands and one monoanionic guanidinate ancillary ligand [(NiPr)2C(NPh2)]. The reaction of the bis(C⁁N) iridium(III) chloride [(C⁁N)2Ir(μ-Cl)]2 with the lithium salt of guanidine ligand [Li{(NiPr)2C(NPh2)}] at 80 °C gave a 65–85% yield of the corresponding heteroleptic [(C⁁N)2Ir{(NiPr)2C(NPh2)}] complexes with several different cyclometalated (C⁁N) ligands such as 2-phenylpyridine (ppy) (1), 2-(2,4-diflurophenyl)pyridine (dfppy) (2), 2-(p-tolyl) pyridine (tpy) (3), benzoquinoline (bzq) (4), 2-phenylbenzoxazole (box) (5), 2-phenylbenzothiazole (btz) (6), 2-(2′-benzothienyl)pyridine (btp) (7) and 1-phenylisoquinoline (piq) (8). These heteroleptic cyclometalated (C⁁N) iridium(III) complexes showed intense absorption bands in the UV region, assignable to ligand-centered (π–π*) transitions and lower energy absorption bands that extended to the visible region are mainly derived from spin-forbidden ligand-centered (π–π*) transitions, as well as metal-to-ligand charge transfer (MLCT) transitions. These complexes also showed intense emissions at room temperature, leading to λmax values from green (λ = 505 nm) to a perfect red colour (λ = 655 nm) with quantum yields (Φ) of 0.18 to 0.64 and phosphorescence lifetimes of 0.78 to 5.80 μs. Organic light-emitting diodes (OLEDs) were fabricated by the use of these complexes as phosphorescent dopants in various concentrations (5–100%) in a N,N′-dicarbazolylbiphenyl (CBP) host. High current efficiency (ηc; up to 125 cd A−1) and power efficiency (ηp; up to 43.6 lm W−1) were observed at appropriate conditions. Because of the steric hindrance of guanidinate ancillary ligands, no significant intermolecular interactions were observed in these complexes, thus leading to the reduction of self-quenching and triplet–triplet (T–T) annihilation at high luminance/currents in OLEDs.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...