Impact of alkaline metal ions Mg2+, Ca2+, Sr2+ and Ba2+ on the structural, optical, thermal and antibacterial properties of ZnO nanoparticles prepared by the co-precipitation method†
Abstract
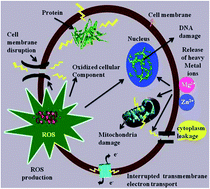
Pure ZnO and alkaline metal ion (Mg2+, Ca2+, Sr2+ and Ba2+)-doped ZnO nanoparticles (NPs) were synthesized by the co-precipitation method. The synthesized nanoparticles retained the wurtzite hexagonal structure, which was confirmed by X-ray diffraction studies. The micro-strain properties were analyzed through Williamson–Hall analysis. The oxidation states of the elements (C (1s), O (1s), Zn (2p), Mg (1s), Ca (2p), Sr (3d) and Ba (3d)) were confirmed by XPS studies. HRSEM studies showed a reduction in the thickness of the ZnO nanoflakes from 63 to 47 nm after doping. EDAX studies determined the amount of dopant (alkaline metals) incorporated into the doped samples. The FT-IR spectra confirmed the Zn–O stretching bands at 432, 416, 414, 426 and 422 cm−1 for the respective ZnO NPs. The photoluminescence measurements revealed that the broad emission was composed of six different bands due to zinc and oxygen vacancies. Thermal analysis revealed that the irreversible structural transition occurred from the cubic phase to the wurtzite phase in the samples. The antibacterial studies performed against a set of bacterial strains showed that the Mg-doped ZnO NPs possessed a greater antibacterial effect than the other alkaline metal ion-doped ZnO NPs.

 Please wait while we load your content...
Please wait while we load your content...