Synthesis, conductivity and structural aspects of Nd3Zr2Li7−3xAlxO12
Abstract
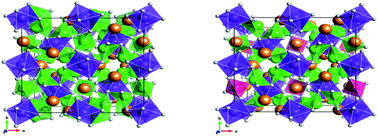
In this paper we report the synthesis, structure and Li ion conductivity of a new tetragonal garnet phase Nd3Zr2Li7O12. In line with other tetragonal garnet systems, the Li is shown to be ordered in the tetrahedral and distorted octahedral sites, and the Li ion conductivity is consequently low. In an effort to improve the ionic conductivity of the parent material, we have also investigated Al doping to reduce the Li content, Nd3Zr2Li5.5Al0.5O12, and hence introduce disorder on the Li sublattice. This was found to be successful leading to a change in the unit cell symmetry from tetragonal to cubic, and an enhanced Li ion conductivity. Neutron diffraction studies showed that the Al was introduced onto the ideal tetrahedral garnet site, a site preference also supported by the results of computer modelling studies. The effect of moisture on the conductivity of these systems was also examined, showing significant changes at low temperatures consistent with a protonic contribution in humid atmospheres. In line with these observations, computational modelling suggests favourable exchange energy for the Li+/H+ exchange process.

 Please wait while we load your content...
Please wait while we load your content...