A chromium oxide solution modified lithium titanium oxide with much improved rate performance
Abstract
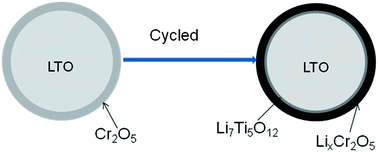
A simple surface modification of Li4Ti5O12 powder is made with an aqueous CrO3 solution to improve its electrochemical properties. At a high current rate of 30 C, the specific capacity of the Li4Ti5O12 increases by about 60% from its original 80 to 130 mA h g−1. After the modification with the CrO3 solution, a few surface phases including lithium chromate (Li2CrO4), Cr2O5 and anatase are found to co-exist in the final product. Among these three phases, Li2CrO4 and Cr2O5 can improve while anatase deteriorates the rate performance of Li4Ti5O12. The AC impedance spectra and electron spin resonance (ESR) spectra reveal that the improvement of rate performance is correlated with the presence of Ti3+, which can increase the conductivity of the Li4Ti5O12 sample. Lithium chromate (Li2CrO4) and Cr2O5 react with lithium to form lithium rich phases (Li3+xCrO4 and LiyCr2O5) of low potential, which stabilize Li7Ti5O12 with high electric conductivity and result in better rate performance. Besides improved rate performance, the cycle performance of the modified Li4Ti5O12 samples is almost at the same level with the original Li4Ti5O12, suggesting that this proposed modification method is efficient and harmless.

 Please wait while we load your content...
Please wait while we load your content...