Role of transition metal nanoparticles in the extra lithium storage capacity of transition metal oxides: a case study of hierarchical core–shell Fe3O4@C and Fe@C microspheres†
Abstract
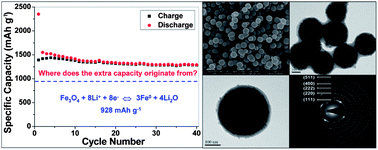
The conversion reaction mechanism has widely been accepted in interpreting and evaluating the lithium storage capability of transition metal oxides (MOs). However, this mechanism cannot well explain the phenomenon of the extra capacity which exists in almost all MO materials and attracts much attention. Up to now, the extra capacity phenomenon has generally been ascribed to the reversible conversion of polymeric gel-like films. However, the essential role of metal nanoparticles in this process has not been systematically investigated. To further illustrate the role of metal nanoparticles for the extra capacity, Fe3O4@C and Fe@C monodispersed hierarchical core–shell microspheres were designed and adopted as the case study. Naturally Fe3O4@C composites exhibited a large Li storage capacity beyond its theoretical value. However, Fe@C microspheres, which are usually regarded to be inert for lithium storage, still presented a certain electrochemical capacity. Fe nanoparticles might serve as electrocatalysts for the reversible conversion of some components of solid electrolyte interface films, and bring extra capacity to Fe3O4 and electrochemical capacity to Fe. This study can enlighten us for the exploiting of advanced active materials and electrolytes for Li ion batteries, and new energy storage devices.

 Please wait while we load your content...
Please wait while we load your content...