Effects of architecture on the electrochemistry of binder-free inverse opal carbons as Li–air cathodes in an ionic liquid-based electrolyte
Abstract
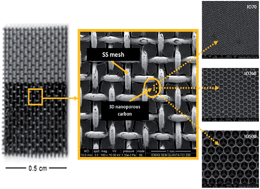
In this work, different electrodes consisting of a layer of nanostructured binderless carbon supported on a stainless steel (SS) mesh have been developed and tested as cathodes for Li–air batteries. Inverse opal (IO) carbons were developed using poly(styrene-co-methacrylic acid) (PS-MAA) spheres of different sizes as templates via a resorcinol–formaldehyde sol–gel process. The resulting electrodes, which were mechanically stable and easy to manipulate, were electrochemically tested at both 25 and 60 °C by galvanostatic cycling in an ionic liquid-based electrolyte (0.3 M LiTFSI in PYR14TFSI). Different ratios of co-monomers used in the preparation of the template polymeric spheres to control their size significantly influenced the resulting surface area, pore volume and pore distribution in IO carbons of different macropore size. From the electrochemical characterisation, transverse trends in reversibility and rate capability were identified depending on the macropore size of the inverse opal carbon. Smaller pores favor a better charge–discharge reversibility. Large pores contribute to an improved rate capability and large capacity, which are likely due, respectively, to deeper oxygen diffusion into the electrode, and to larger pore bottlenecks.

 Please wait while we load your content...
Please wait while we load your content...