An all-solid-state rechargeable aluminum–air battery with a hydroxide ion-conducting Sb(v)-doped SnP2O7 electrolyte†
Abstract
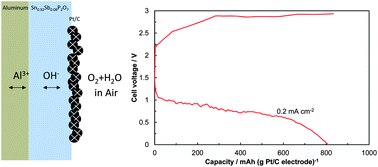
An anhydrous hydroxide ion conductor, Sn0.92Sb0.08P2O7, can function as an electrolyte for aluminum–air batteries, where aluminum is oxidized to an aluminate species during discharge, and the aluminate species is reduced to aluminum by charging the cell. This battery generated an open-circuit voltage of ca. 1.6 V with a discharge capacity of ca. 800 mA h g−1electrode.

 Please wait while we load your content...
Please wait while we load your content...