Fabrication of a new MgO/C sorbent for CO2 capture at elevated temperature†
Abstract
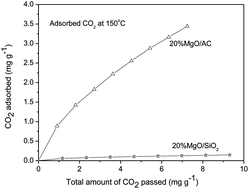
A synergistic effect between a self-dispersion of MgO and biomass-derived carbon in the adsorption of CO2 is reported for the first time. Magnesia and carbon mixed particles are formed in situ during the carbonization of magnesium acetate on activated carbon made from coconut, increasing the accessibility of basic sites for CO2 adsorption. With these complementary effects of the hierarchical structural support, the composite containing 20% magnesia can trap 5 times more CO2 than 20% MgO/SiO2, in the harsh instantaneous adsorption of CO2 at 100 and 150 °C, offering a new candidate for the adsorption of CO2 in flue gas vents.

 Please wait while we load your content...
Please wait while we load your content...