Improved oxygen reduction reaction activities with amino acid R group functionalized PEG at platinum surfaces†
Abstract
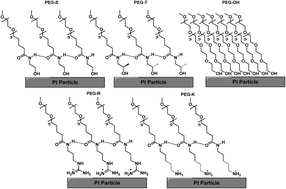
We demonstrate here that coupling specific amino acid “R” groups to polyethylene glycol (PEG) chains through amide bonds and adsorbing them to platinum (Pt) black improves oxygen reduction reaction (ORR) catalysis over a bare metal, even at high polymer loadings. Through our studies, we first show that the presence of PEG on the Pt nanoparticles increases half-wave (E1/2) potentials, most likely by preventing Pt oxidation or Pt–OH formation. We also show, however, that increases in E1/2 did not necessarily correlate with gains in mass activities, and that while commercial PEG–OH in fact lowered mass activities, conjugation of PEG to alcohol-containing amino acid R groups connected by amide bonds led to 100–200% gains in reactivity. This work presents evidence that counters the common conception that organic capping ligands decrease catalytic activity; in fact activity may actually be improved over the bare metal through judicious choice and design of ligands that inhibit Pt oxidation and control chain packing at the Pt surface. These studies therefore show that it may still be possible to have ligands on a nanoparticle surface that allow the particles to be well-dispersed on an electrode surface while simultaneously enhancing catalysis without further particle treatment.

 Please wait while we load your content...
Please wait while we load your content...