Oxygen reduction and transport on the La1−xSrxCo1−yFeyO3−δ cathode in solid oxide fuel cells: a first-principles study†
Abstract
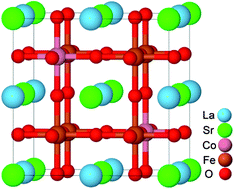
Oxygen reduction and successive migration on a cathode are key steps in solid oxide fuel cells. In this work, we have systematically studied the adsorption, dissociation, incorporation, and successive diffusion of oxygen species on the La1−xSrxCo1−yFeyO3 (LSCF) cathode on the basis of density-functional theory calculation. We found that the O2 molecule prefers to be adsorbed on the transition metal atoms at the B site (Fe or Co) than those at the A site (La or Sr). The oxygen molecule forms either superoxide (O2−) or peroxide (O22−) species on the surface transition metal atoms, and the isomerisation energy barrier energies between them are less than 0.14 eV. The SrCo-terminated surface has the smallest oxygen vacancy formation energy, and the existence of surface oxygen vacancy promotes the oxygen dissociation on the B-site atom without an energy barrier. Instead, without the surface oxygen vacancy, the oxygen dissociation on the Co site needs to overcome an energy barrier of 0.30 eV, while that on the Fe site is about 0.14 eV. The calculated minimum energy pathways indicate that the energy barrier of oxygen migration on the surface is much higher than that in the bulk which contains the oxygen vacancy. Moreover, increasing the concentration of Co will effectively facilitate the formation of oxygen vacancy, greatly enhancing the oxygen bulk transport. Our study presents a comprehensive understanding of the mechanism of oxygen reduction and migration on the LSCF cathode.

 Please wait while we load your content...
Please wait while we load your content...