Hierarchically order porous lotus shaped nano-structured MnO2 through MnCO3: chelate mediated growth and shape dependent improved catalytic activity†
Abstract
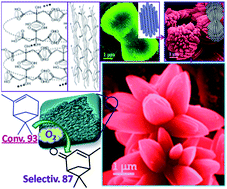
Design of hierarchical nanostructures towards a specific morphology is an important research area due to their shape dependent properties. Here, 3D hierarchically assembled lotus shaped porous MnO2 is synthesized using a simple aqueous solution based chelating agent (citric acid) mediated growth of MnCO3 followed by calcination at 350 °C. MnCO3 in other shapes, such as rods, spheres and nano-aggregates, is also synthesized just by varying the chelating agents. It is observed that the geometry and strength of the chelating ligands has a crucial role in the controlled shape selective synthesis and based on this a probable chelating agent driven formation mechanism is discussed. The synthesized porous MnO2 shapes exhibit excellent shape dependent catalytic oxidation of α-pinene to verbenone using molecular oxygen as the oxidant. The lotus shaped porous MnO2 shows superior activity, with 94% conversion of α-pinene and 87% selectivity of verbenone, to that of other MnO2 shapes. The activity is reasonably high compared to heterogeneous as well as homogeneous catalysts reported in the literature and bulk MnO2 with respect to both their conversion and selectivity. The synthesized lotus shaped MnO2 also showed good catalytic activity towards oxidation of allylic compounds to corresponding ene–ones using molecular oxygen as oxidant and is reusable.

 Please wait while we load your content...
Please wait while we load your content...