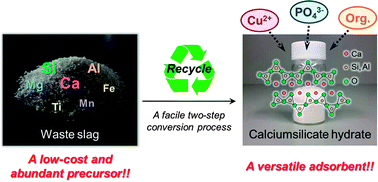
Blast furnace slag (BFS), a high volume byproduct resulting from the iron manufacturing industry, was used as a low-cost and abundant precursor for preparing a high-surface-area calcium silicate hydrate. The synthesis of slag-made calcium silicate hydrate (slagCS) was achieved via a facile two-step dissolution–coprecipitation procedure using HCl and NaOH, respectively. The use of concentrated protonic acid facilitated an easy dissolution of BFS, and the subsequent addition of aqueous NaOH solution at 100 °C yielded a hydrate product with over 85% atom economy (10.9 g from 10.0 g of BFS). By the combination of XRD, FT-IR, 29Si MAS NMR, FE-SEM, nitrogen physisorption and chemical analysis, the product synthesized under optimum conditions was identified to be a calcium silicate hydrate with a stoichiometry of Ca : Si = 1 : 1 and with a mean surface area of 219 m2 g−1 incorporating the slag-derived metals (Al, Mg, Fe, Ti and Mn) in its structure. The thus synthesized slagCS showed good adsorption properties for removal of Cu2+, phosphate ions and model protein (ovalbumin) diluted in water, making this material a promising candidate for efficient bulk wastewater treatment.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...