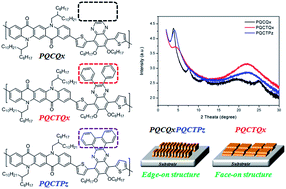
In this study, a highly soluble poly[quinacridone-alt-quinoxaline] series (PQCQx, PQCTQx, PQCTPz) was synthesized through the Suzuki coupling reaction by introducing planar quinacridone and highly soluble quinoxaline. The polymers were soluble in general organic solvents, and the Mn was 15.6–85.0 kg mol−1. The optical band gap energy was 1.82–1.97 eV, which was similar to the band gap of a benzothiadiazole derivative. The HOMO and LUMO levels of the polymers were −5.32 to −5.46 eV and −3.40 to 3.50 eV, respectively. According to XRD measurements, the PQCQx and PQCTPz showed the formation of an ordered lamellar structure and conventional edge-on π-stacking, while the PQCTQx showed face-on formation relative to the substrate. This study also evaluated the OPV characteristics by fabricating a bulk-heterojunction-type polymer solar cell. For the device structure of ITO/PEDOT:PSS/active layer (PQCTQx:PC71BM = 1 : 2 with DIO)/PFN/Al, the values of open-circuit voltage (VOC), short-circuit current (JSC), fill factor (FF) and power conversion efficiency (PCE) were 0.85 V, 7.6 mA cm−2, 54.9%, and 3.6%, respectively.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...