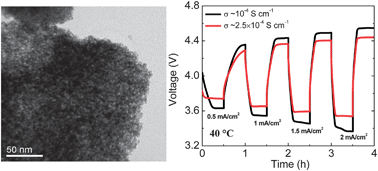
Two major issues limiting the conversion efficiency and power density of dual-electrolyte Li–air cells are the lack of efficient oxygen evolution catalysts and high internal resistance associated with the solid electrolyte. In this context, the charge voltage is lowered by 0.11 V at a charge current density of 2 mA cm−2 by employing nanocrystalline IrO2 synthesized by a modified Adams fusion method. Similarly, the overall internal resistance of the cell is reduced substantially by increasing the operating temperature of the cell from 20 to 40 °C, resulting in a nearly three-fold increase in the maximum power density. Overall, the conversion efficiency at 2 mA cm−2 is improved from 61% to 74% at 40 °C with the nanocrystalline IrO2. The internal resistance is further reduced by employing a more conductive solid electrolyte at 40 °C, resulting in a maximum power density and conversion efficiency at 2 mA cm−2 of, respectively, 40 mW cm−2 and 80%.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...