Electrochemically reduced graphene oxide multilayer films as metal-free electrocatalysts for oxygen reduction†
Abstract
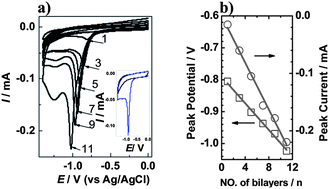
In this work we report on functional multilayer films containing electrochemically reduced graphene oxide (ERGO) by the alternating layer-by-layer (

 Please wait while we load your content...
Please wait while we load your content...