Altered reaction pathways of eutectic LiBH4–Mg(BH4)2 by nanoconfinement†
Abstract
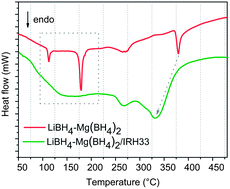
The effects of nanoconfinement on the dehydrogenation rate and reaction pathways of the eutectic LiBH4–Mg(BH4)2 have been comprehensively investigated. By means of thermal analysis,

 Please wait while we load your content...
Please wait while we load your content...