High-temperature proton exchange membranes from microporous polyacrylamide caged phosphoric acid
Abstract
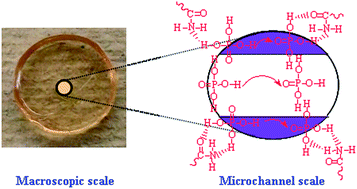
To enhance the H3PO4 loading and therefore proton conductivity, freeze-dried microporous polyacrylamide (PAM) was doped with a highly concentrated H3PO4 aqueous solution, leading to high percentages of cages of H3PO4 in the PAM membranes for potential use in high-temperature proton exchange membranes (PEMs). A H3PO4 fraction of 92.0 wt% and a proton conductivity as high as 0.132 S cm−1 at 183 °C were obtained. The H3PO4 loading by microporous PAM membranes obeys a Fickian diffusion process. Protons migrate along the channels from the H3PO4 bonded PAM framework or P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H–O hydrogen bonds of H3PO4 molecules according to the Grotthuss mechanism. This new concept, in combination with high conductivity, good H3PO4 retention and simple-synthesis, opens a new approach to high-temperature PEMs.
O⋯H–O hydrogen bonds of H3PO4 molecules according to the Grotthuss mechanism. This new concept, in combination with high conductivity, good H3PO4 retention and simple-synthesis, opens a new approach to high-temperature PEMs.

 Please wait while we load your content...
Please wait while we load your content...