Rate of gas absorption on a slippery bubble mattress
Abstract
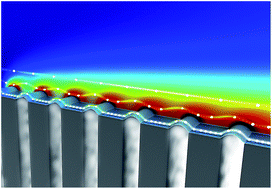
We investigate the absorption of a pure gas into a liquid in laminar flow past a superhydrophobic surface consisting of alternating solid walls and micro-bubbles. We experimentally measure and numerically estimate the dynamic mass transfer of gas absorption at stable gas–liquid interfaces for short contacting times. We study the net rate of gas absorption experimentally by in situ measurements of dissolved oxygen concentration profiles in aqueous solutions flowing over oxygen bubbles by fluorescence lifetime imaging microscopy. We numerically analyze the dynamics of interfacial mass transfer of dissolved oxygen by considering (i) kinetic equilibrium conditions at bubble surfaces that are conventionally described by Henry's Law and (ii) non-equilibrium conditions at bubble surfaces using Statistical Rate Theory (SRT). Our experimental results show that kinetic equilibrium is not established for short contact times. Mass transfer of gas into liquid flow past micro-bubbles can be well described by our simulations performed with the non-equilibrium theory for a short exposure time (∼180 μs) of liquid with a microbubble, deviating from the commonly accepted Henry's Law.

 Please wait while we load your content...
Please wait while we load your content...