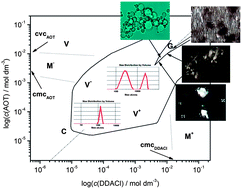
Phase transitions from a dispersed lamellar to hexagonal liquid crystalline phase have been investigated in a catanionic surfactant mixture formed by mixing a single tailed cationic surfactant, dodecylammonium chloride, with a double tailed anionic surfactant, sodium bis(2-ethylhexyl) sulfosuccinate. Depending on the bulk composition and total surfactant concentration, mixed micelles, vesicles, lamellar and hexagonal columnar liquid crystalline phases have been identified. Differences in the geometry of the two hydrophobic chains stabilize vesicles of different shapes (spherical, tubular and pearled) relative to the liquid crystalline phase even in stoichiometric mixtures. At higher surfactant concentrations the phase transition from a dispersed lamellar to hexagonal columnar liquid crystalline phase proceeds continuously, with both phases coexisting over a range of concentrations. The transition proceeds through processes of vesicle aggregation, reorganization into multilayer sheets rolled-up into tubules, and formation of a hexagonal columnar liquid crystalline phase.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...