The hydrophilic/hydrophobic nature of a Cl-terminated Si surface
Abstract
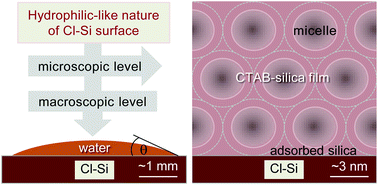
The hydrophilic/hydrophobic nature of a Cl-terminated Si surface, which is slightly controversial, was investigated on the molecular level by depositing CTAB–silica 2D-hexagonal mesostructured films on it as well as on OH- and H-terminated Si surfaces and determining their structures by combining X-ray reflectivity and grazing incidence small angle X-ray scattering measurements. The contact angle measurements, which provide macroscopic level information, confirm that the Cl–Si surface is hydrophilic in nature but less hydrophilic than the OH–Si surface. The initial attachment of a film (through a silica layer) and the (near circular) shape of the micelles, which provide microscopic level information, clearly indicate that the Cl–Si surface is hydrophilic in nature and its hydrophilicity is comparable to that of the OH–Si surface. The relatively strong electronegativity of Cl is probably the reason for the hydrophilic nature of the Cl–Si surface, while the lack of hydrogen bonding and/or the lack of complete chlorination are a hindrance.

 Please wait while we load your content...
Please wait while we load your content...