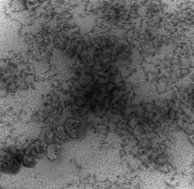
We investigated the temperature-induced liquid–liquid phase separation (coacervation) of polyelectrolyte (PE)–micelle systems and the structure of the resultant coacervates. Dynamic light scattering, small angle neutron scattering and cryo-transmission electron microscopy (DLS, SANS, Cryo-TEM) were used to examine the evolution of complex structure up to the point of temperature-induced coacervation and beyond. Three diffusional modes, seen in the single phase samples and in the coexisting coacervated supernatant phases were attributed respectively to free micelles, PE–micelle complexes, and aggregates thereof. They corresponded to SANS Guinier region slopes yielding Rg ∼ 4 nm (micelles) and Rg ∼ 50 nm (unresolved complexes and aggregates). Cryo-TEM images of coacervates indicated how these subunits are organized within dense and dilute coacervate domains at larger length scales. Taken together, these results are understood to arise from the requirements of overall charge neutralization, and ion-pairing and counterion release during coacervation. We conclude that a polyelectrolyte:micelle system at incipient coacervation with charge stoichiometry ([+]/[−] > 1) donates excess polycations to other complexes in solution. In the coacervate, a similar disproportionation but at different length scales ejects excess polycations and their counterions into dilute domains. In both phases, association and desolvation are driven by counterion release, enhanced chain configurational entropy, and ion-pairing. These enthalpic and entropic forces operating in both phases could explain the structural similarities between soluble aggregates and coacervate dense domains.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...