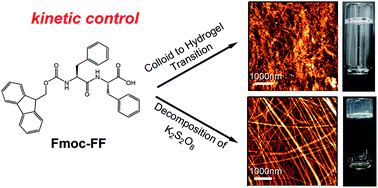
Small-molecule hydrogelators have been widely used to engineer supramolecular hydrogels for biomedical applications. Typically, a change of the solubility of small molecules in solvent is used to trigger the gelation process. This requires a switch of pH or solvent by mixing two different types of solutions. However, due to the intrinsic ragged free energy landscape that underlies the self-assembly process and the high viscosity of the solution that limits the diffusion, the hydrogels made by these methods are often limited by their inhomogeneity and irreproducible physical properties. It is therefore desirable to circumvent these drawbacks and produce homogeneous hydrogels. Conversely, only a few studies have been done towards this direction. In this article, we present two novel approaches to engineer homogeneous hydrogels. One is based on the nano-dispersed colloids to hydrogel transition and the other is based on the decomposition of potassium persulfate to mildly change the pH. These two methods allow kinetically controlling the self-assembly process and the resulting hydrogels are indeed more homogeneous and reproducible. Moreover, the structural and morphological characterizations suggest that the structures of the hydrogels prepared by different approaches are distinct from each other, leading to diverse macroscopic mechanical properties. These results suggest that besides the thermodynamics, the self-assembly kinetics also plays an important role in determining the properties of the final assembled hydrogels. We propose that it is possible to rationally tune the physical properties of the hydrogels by simply control the self-assembly kinetics without changing the structure of the small-molecule hydrogelators.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...