Self-consistent field theory study of the solvation effect in polyelectrolyte solutions: beyond the Poisson–Boltzmann model†
Abstract
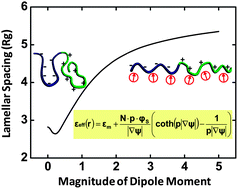
We developed a self-consistent field theory to study the solvation effect in polyelectrolyte solutions by taking into account the dipolar feature of
 Please wait while we load your content...
Please wait while we load your content...