Dual stimuli-responsive supramolecular pseudo-polyrotaxane hydrogels†
Abstract
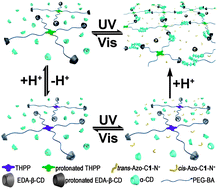
THPP-(PEG2000-BA)4, a four-branched molecule end-decorated with benzaldehyde (BA), was successfully designed and synthesized. It can form physical pseudo-polyrotaxane (PPR) hydrogels in the presence of α-cyclodextrins (α-CDs). The branched structure of the THPP core promotes gel formation with a small amount of host and guest. Moreover, these resulting hydrogels are dual stimuli-responsive, which can be observed by physically macroscopical phenomena and 1H NMR spectra. Since BA can react with amine to form a pH-responsive Schiff-base which possesses a dynamic covalent nature, it is anticipated that the formation of gels can be modulated by pH. When THPP-(PEG2000-BA)4 was blocked by the relatively large molecule 6-N-ethylenediamine-6-deoxy functionalized β-CD (EDA-β-CD) gelation did not take place. However, the addition of an acid resulted in gelation since the benzoic imine bonds can hydrolyze under acidic conditions and α-CDs can thus pass through BA to thread on the polyethylene glycol (PEG) chains. When a strongly competitive guest, 1-[p-(phenylazo)benzyl]-bromide (Azo-C1-N+), was added to the gel, a gel-to-sol transition was observed due to the disassembly of inclusion complexes between α-CDs and PEG chains. Simultaneously, this gel shows a photo-responsive capacity because of the presence of azobenzene. Therefore, the cycles of gel–sol transitions were achieved through pH- and photo-stimuli. This kind of hydrogel is promising for use in many fields, such as biology and electronics.

 Please wait while we load your content...
Please wait while we load your content...