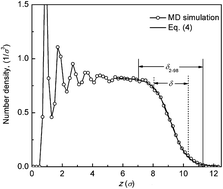
Understanding the kinetics of lubricant depletion under rapid and isothermal heat treatment is of fundamental importance in maintaining the stability and reliability of lubricated surfaces. In this paper, molecular dynamics simulation coupled with a coarse-grained bead-spring polymer model is employed to study lubricant depletion instability. During rapid heating, the lubricant decomposes at an increasing rate with temperature. However, a maximum rate is observed during desorption due to the influence of lubricant-to-substrate interaction. During the isothermal stage, the rates of desorption (rdes) and decomposition (rdec) decrease over time, showing an exponential depletion process. It is found that rdes or rdec increases with both the temperature and lubricant mass or bond coverage. Moreover, the rate constants for desorption (kdes) and decomposition (kdec) are calculated based on a first-order kinetics-controlled reaction. For a given lubricant mass coverage, ln(kdes) exhibits a non-linear relation with 1/T, indicating non-Arrhenius-like behavior of lubricant desorption. However, for a given lubricant bond coverage, ln(kdec) versus 1/T yields a straight line, signifying Arrhenius-like behavior of lubricant decomposition. The kinetics of lubricant depletion also shows that lubricant desorption is favored over decomposition under heat treatment to high temperatures and is the major cause of lubricant degradation and failure on the surface.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...