Accessing extended and partially fused hexabenzocoronenes using a benzannulation–cyclodehydrogenation approach†
Abstract
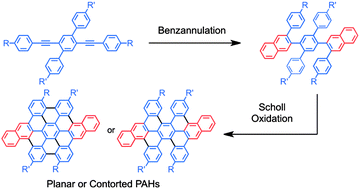
A rapid and efficient approach to prepare extended or partially fused hexabenzocoronene derivatives is described. The method is based on the sequential benzannulation and cyclodehydrogenation (Scholl

 Please wait while we load your content...
Please wait while we load your content...