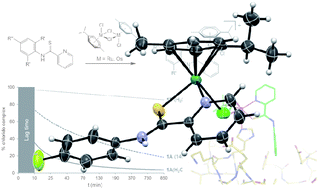
A novel series of organometallic antitumour agents based on RuII and OsII complexes containing N-substituted 2-pyridinecarbothioamides (PCAs) has been synthesized and characterized. To the best of our knowledge, this is the first report of organometallic anticancer compounds with an S,N-bidentate ligand system. While the ligands showed activity as gastric mucosal protectants and low acute toxicity in vivo (J. Med. Chem., 1990, 33, 327–336), coordination leads to highly antiproliferative metallodrugs, depending on lipophilicity and steric demand, in colon carcinoma and non-small lung cancer cell lines with intrinsic chemoresistances. The most lipophilic and smallest congeners are the most effective with IC50 values in the low micromolar range. This new family of potential metallodrugs features exceptional stability in hydrochloric acid (60 mM), characterized by complete suppression of hydrolysis and low reactivity towards biological nucleophiles. Therefore, their unexpected aqueous chemistry renders this family of antiproliferative agents suitable for oral administration. An unprecedented feature is their ability to form transient thioketone-bridged dimers in aqueous solution upon hydrolysis, which is believed to minimize deactivation by biological nucleophiles. However, the biological effect seems to be caused by the monomer as observed with crystallographic studies of the nucleosome core particle (NCP), which revealed that [chlorido(η6-p-cymene)(N-phenyl-2-pyridinecarbothioamide)osmium(II)] chloride and [chlorido(η6-p-cymene)(N-fluorophenyl-2-pyridinecarbothioamide)osmium(II)] chloride react at two types of binding sites on the histone proteins. The adducts form at histidine side chains located on the nucleosome surface and the inner cleft of the nucleosome in the midst of an extensive histone–histone interface, suggesting interference with chromatin activity as a possible mode of action of these compounds. Additionally, ligand-based S → O exchange allows for a potential dual-mode of action by targeting DNA (J. Med. Chem., 2009, 52, 7753–7764). The quantitative estimates of drug-likeness (QED) for this family of compounds revealed a similar drug-likeness compared to erlotinib, tamoxifen, imatinib and sorafenib.

 Please wait while we load your content...
Please wait while we load your content...