Lewis-base silane activation: from reductive cleavage of aryl ethers to selective ortho-silylation†
Abstract
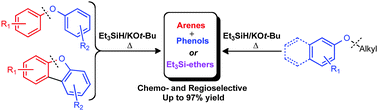
We report a transition-metal-free protocol for the efficient reductive cleavage of diaryl and aryl alkyl ethers. The combination of triethylsilane with common bases forms an unusually powerful reductive couple that regioselectively ruptures lignin- and coal-related C–O bonds in aromatic ethers. Interestingly, with certain bases and temperature regimes ortho-directed C–H silylation efficiently competes with the latter process. However, careful tuning of the reactions conditions allows for the selective reductive cleavage of lignin model compounds to their corresponding phenolic and aromatic constituents.

 Please wait while we load your content...
Please wait while we load your content...