Analysis of supramolecular complex energetics in artificial replicators†
Abstract
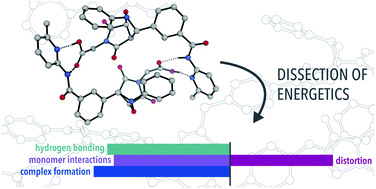
The energetics of complex formation in a number of artificial self-replicating systems has been studied by density functional theory calculations. Complex stabilities were dissected into stabilizing contributions from hydrogen bonding, dispersion, electrostatics, and destabilizing distortions of monomers from their optimum geometries. Strong cooperative effects were identified in all systems, and dispersion and electrostatics contribute more to the overall energies of complex formation than hydrogen bonding in some systems.

 Please wait while we load your content...
Please wait while we load your content...