A radical-based approach for the construction of the tetracyclic structure of resiniferatoxin†
Abstract
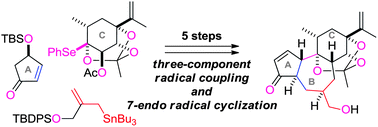
A novel radical-based strategy for accessing the unique tetracyclic skeleton of resiniferatoxin is described. The synthetic route is characterized by a stereoselective synthesis of the C-ring which has a bridgehead O,Se–acetal, a three component radical coupling of the A- and C-rings and a branched allyl stannane, and a 7-endo radical cyclization to construct the fused B-ring from the coupling adduct. The present approach attests to the power of radical reactions to realize the congested C–C bond formations required for synthesizing highly functionalized compounds.

 Please wait while we load your content...
Please wait while we load your content...