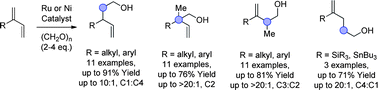
The regioselective reductive coupling of paraformaldehyde to 2-substituted dienes at positions C1, C2 and C3 was achieved using metal catalysts based on nickel, cationic ruthenium and neutral ruthenium, respectively. Whilst C1 adducts predominate using dienes, silicon- or tin-substituted dienes exhibit C4 regioselectivity – the first time that this regioselectivity has been observed for the coupling of 2-substituted butadienes to aldehydes. Both nickel- and ruthenium-catalyzed processes avoid pyrophoric or mass intensive reducing agents, using paraformaldehyde or isopropanol, respectively. These couplings may be viewed as alternatives to diene hydroformylation, for which regioselective formation of constitutionally isomeric products has not yet been achieved.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...