Redox regulation of tumour suppressor protein p53: identification of the sites of hydrogen peroxide oxidation and glutathionylation†
Abstract
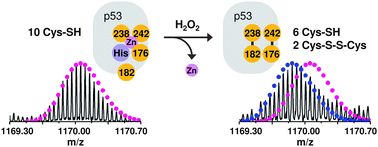
The p53 tumour suppressor protein promotes cell cycle arrest or apoptosis in response to several forms of cellular stress and DNA damage. p53 acts as a transcription factor by binding to specific DNA sequences and regulating the expression of a myriad of target genes. p53 activity is known to be influenced by several post-translational modifications and is dependent on coordination of a zinc ion within the protein core domain. Several lines of evidence have demonstrated that oxidative modification of cysteine residues within p53 can also influence the protein's activity. However, little is known regarding the molecular details of p53 oxidation. Here we analyze oxidation pathways in the p53 core domain by Fourier transform ion cyclotron resonance mass spectrometry and top-down fragmentation. Firstly, we show that p53 core domain is sensitive to oxidation by the reactive oxygen species (ROS) hydrogen peroxide and that the zinc-coordination site is the initial target for ROS-induced oxidation. Two disulfide bonds are formed involving Cys182 and the three cysteines which coordinate to zinc (Cys176, 238 and 242). This disulfide bond formation is accompanied by loss of zinc from the binding site. Secondly, an additional cysteine, Cys277, is prone to oxidation via a ROS-independent mechanism. This residue undergoes S-glutathionylation at biologically relevant reduction potentials. We discuss our findings in the context of redox regulation of p53 activity and in comparison to other redox regulated proteins.

 Please wait while we load your content...
Please wait while we load your content...