Divergent reaction pathways for phenol arylation by arynes: synthesis of helicenes and 2-arylphenols†
Abstract
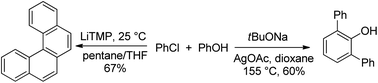
Two reactions of phenols with arynes have been developed. If LiTMP base is employed, arynes generated from aryl chlorides react with phenols to form helicenes. o-Arylation of phenols can be achieved by employing tBuONa base in the presence of AgOAc. Direct arylation of binol was achieved leading to the shortest pathway to o,o′-diarylbinols.

 Please wait while we load your content...
Please wait while we load your content...