Exploring ionic liquid–biomass interactions: towards the improved isolation of shikimic acid from star anise pods†
Abstract
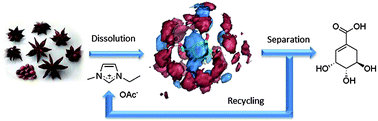
Based on shikimic acid – the starting material for the important anti-influenza drug Tamiflu (oseltamivir phosphate) – we present that the dissolution properties of ionic liquids can lead to better access to the valuable ingredient embedded in the biopolymer. Different imidazolium-based ionic liquids were investigated, and the extraction yield of shikimic acid was correlated with their hydrogen-bonding properties via polarizable MD simulations, indicating that the hydrogen bonding of the IL anion to shikimic acid is responsible for a good extraction performance. A scale-up strategy for the isolation of shikimic acid with the ionic liquid 1-ethyl-3-methylimidazolium acetate ([C2mim]OAc) was developed based on ion-exchange resins, thus allowing isolation of shikimic acid in up to 10 wt% yield with complete recovery of the ionic liquid.
- This article is part of the themed collection: 5th Congress on Ionic Liquids

 Please wait while we load your content...
Please wait while we load your content...