Phase transitions of iron sulphides formed by steel microbial corrosion
Abstract
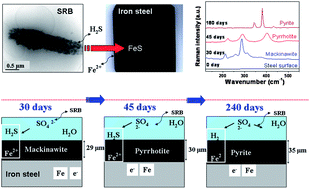
In this study, we investigated the iron sulphides phase transitions resulting from steel corrosion under sulphate-reducing-bacteria conditions. Experiments were conducted at 30 °C from 7 to 240 aging days. Culture batch experiments were conducted with steel coupons, clay and groundwater amended with lactate to simulate steel degradation under anaerobic conditions. Solutions were monitored by inductively-coupled plasma mass spectrometry and ionic chromatography. The corrosion products were investigated by scanning electron microscopy/energy dispersive X-ray spectroscopy, X-ray diffraction and by confocal micro-Raman spectrometry. Microbial activity was evidenced by a drop in sulphate concentration and production of sulphide. In the early stages of corrosion (7 days) poorly crystallised iron sulphide (FeS) was identified, followed by the formation of mackinawite (FeS1−x) and after 30 days, mackinawite evolved into pyrrhotite (FeS1+x) and ultimately to the more stable pyrite (FeS2). The same mineralogical sequence was identified in similar abiotic experiments in the presence of increasing sulphide concentration. The evolution of Raman vibrational spectra was quantitatively examined for the first time and the protective role of the pyrrhotite and pyrite layers with increasing time was discussed.

 Please wait while we load your content...
Please wait while we load your content...